11
2020-12
2020年11月09日,NATURE MEDICINE 杂志(IF:36.13)发表《Rapid pathogen detection by metagenomic next - generation sequencing of infected body fluids》研究文章,探讨新一代宏基因组测序对感染体液快速进行病原检测的应用,该研究入组160名急性病患者,并收集了182种体液进行mNGS检测,并与培养法、PCR、纳米孔测序等方法进行微生物学测试的对比。
我们使用体液中的无细胞DNA进行了mNGS测试,以鉴定病原体。使用两种测序平台对160名急性病患者的182种体液的mNGS测试性能进行了评估,并与使用培养物、16S细菌PCR和/或28S-内部转录核糖体基因间隔子(28S-ITS)真菌PCR的微生物学测试进行对比。通过Illumina测序,细菌的检测灵敏度和检测特异性分别为79%和91%,真菌为91%和89%;通过纳米孔测序,细菌分别为75%和81%,真菌为91%和100%。在12例培养/ PCR阴性体液但最终被确诊为感染的患者中,有7例(58%)是mNGS阳性。实时计算分析可通过中值50分钟测序和6小时样品到应答时间的纳米孔测序实现病原体鉴定。快速的mNGS测试是诊断体液未知感染的潜在工具。
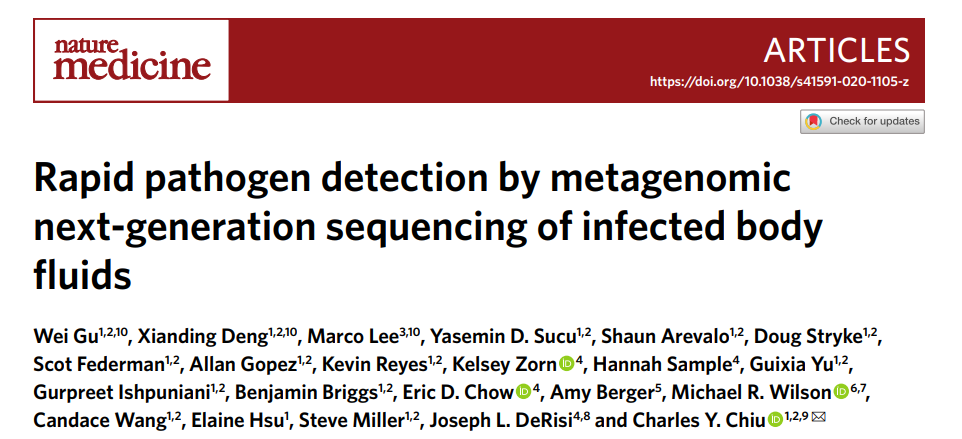
图a:mNGS体液分析工作流程示意图
a, Schematic of mNGS body fluid analysis workflow. The clinical gold standard consisted of aggregated results from cultures, bacterial 16S PCR and/or fungal 28S–ITS PCR, while the composite standard also included confirmatory digital PCR with Sanger sequencing and clinical adjudication. For nanopore sequencing in <6 h, 40–60 min are needed for nucleic acid extraction, 2–2.5 h for mNGS library preparation, 1 h for nanopore 1D library preparation and 1 h for nanopore sequencing and analysis.
(临床金标准由培养物、细菌16SPCR和/或真菌28S-ITSPCR的共同结果,而复核标准还包括与Sanger的验证性数字PCR测序和临床诊断。 对于< 6 h的纳米孔测序,核酸提取需40~60 min,mNGS文库制备需2~ 2.5 h,纳米孔1D 建库需1 h,纳米孔测序分析需1 h。)
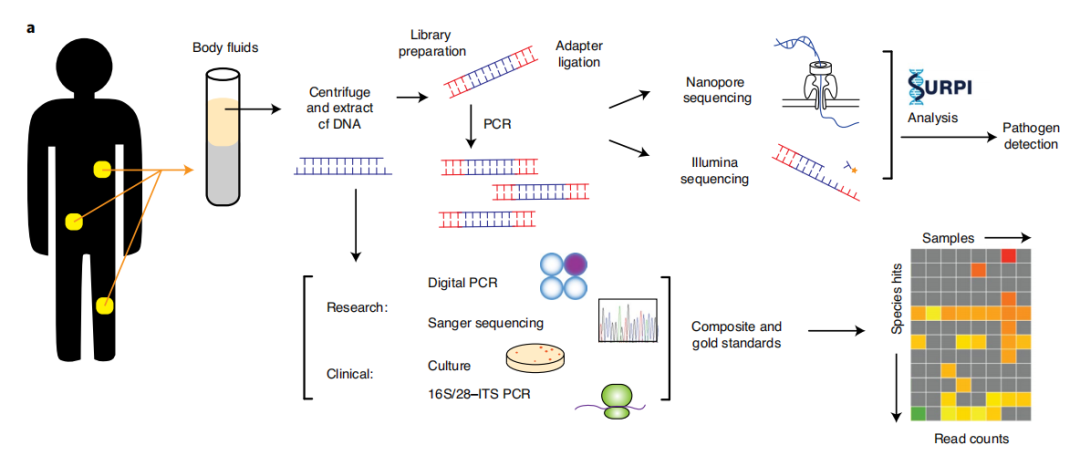
图b:研究中包括的182例体液样本的分析工作流程
b, Analysis workflow for the 182 body fluid samples included in the study. 170 samples were included in the accuracy assessment while 12 samples collected from patients with a clinical diagnosis of infection but negative microbiological testing were included for mNGS analysis. The pie chart displays the body fluid sample types analyzed in the study.
(在准确性评估中包括170个样本,从临床诊断感染的患者中收集的12个样本,但对阴性微生物样本进行了mNGS检测。饼图显示研究中分析的体液样本类型。)
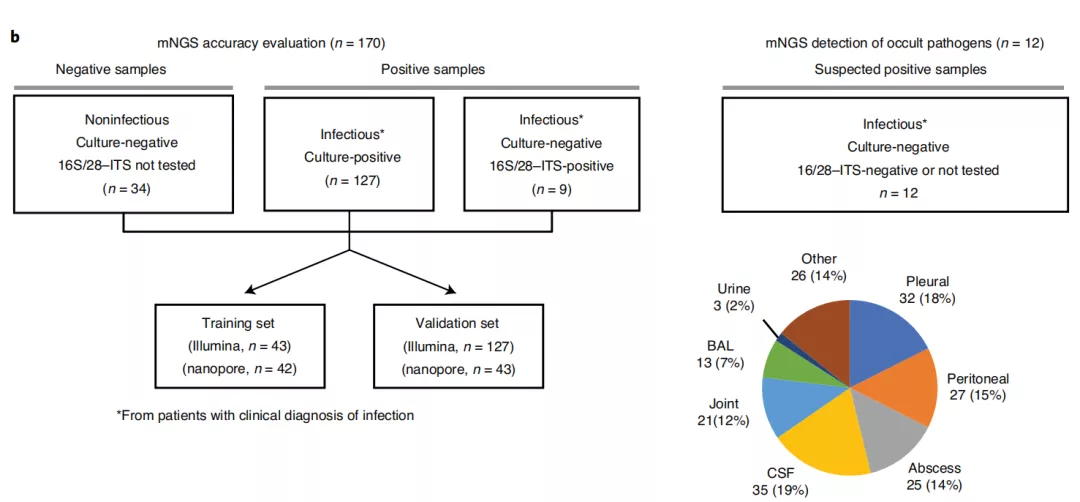
图c:相对于培养的mNGS测试的时机
c, Timing for mNGS testing relative to culture. Whereas culture-based pathogen identification can take days to weeks, mNGS testing using nanopore or Illumina sequencing platforms has an overall turnaround time of 5–24 h.
(基于培养的病原体鉴定可能需要几天到几周,而使用纳米孔或Illumina测序平台进行mNGS测试的总体运行时间为5-24小时。)
参考文献:[1]. Rapid pathogen detection by metagenomic next-generation sequencing of infected body fluids.[J]. Nature medicine,2020.
